WHO IHR Modifications Were Illegally Approved
In blatant disregard for established protocol and procedures, sweeping IHR amendments were prepared behind closed doors, and then both were submitted for consideration and accepted by the World Health Assembly quite literally in the last moments of a meeting that stretched late into Saturday night, the last day of the meeting schedule.
Although the “Article 55” rules and regulations for amending the IHR explicitly require that “the text of any proposed amendment shall be communicated to all States Parties by the Director-General at least four months before the Health Assembly at which it is proposed for consideration,” the requirement of four months for review was disregarded in a rush to produce some tangible deliverable from the Assembly. This hasty and illegal action was taken in direct violation of its own charter, once again demonstrating an arbitrary and capricious disregard of established rules and precedent by the WHO under the leadership of the Director-General.

There was no actual vote to confirm and approve these amendments. According to the WHO, this was achieved by “consensus” among this unelected insider conclave rather than a vote; “Countries agreed by consensus to amend the International Health Regulations, which were last changed in 2005, such as by defining the term “pandemic emergency” and helping developing countries to gain better access to financing and medical products,” a WHO statement reported, continuing that “countries” agreed to complete negotiations on the pandemic accord with the year, “at the latest.”
Representatives from many WHO member nation-states were not in the room, and the ones that were there were encouraged to keep quiet. After the non-vote, there was giddy celebration of this achievement, clearly demonstrating the lack of somber maturity, commitment to both rules and careful diplomatic consensus, and absence of serious intent and purpose warranted by the topic.
This was clearly an insider clique acting unilaterally to circumvent normal process and mirrors a similar process used to confirm the re-appointment of Tedros Ghebreyesus to the Director-General position. This unelected WHO clique of “true believers” clearly signals that it believes itself above any requirements to comply with established international norms and standards, including its own. By their actions you will know them; the giddy arrogance of these actions predicts that WHO decision-making will continue to be arbitrary, capricious, and politicized, and will continue to reflect the will of various insider interest groups (and nation-states) rather than anything even approximating a broad-based international consensus.
Here in the United States, these unilateral actions, backed by an executive branch and bureaucracy that repeatedly demonstrates a deep disdain for the rule of law and the US Constitution, may require that individual States pass legislation to reject the WHO Amendments to IHR based on the illegality of the process and violation of Article 55. Similar discussions are occurring in the UK and across many WHO member states, adding momentum to the emerging WHO-exit movement.
For those not familiar, the current WHO Director-General Tedros Adhanom Ghebreyesus is neither a physician nor a trained public health or epidemiology specialist, but rather is an Ethiopian microbiologist, malaria researcher, and politician.
The hastily approved IHR consolidates virtually unchecked authority and power of the Director-General to declare public health emergencies and pandemics as he/she may choose to define them, and thereby to trigger and guide the allocation of global resources as well as a wide range of public health actions and guidances. These activities include recommendations relating to “persons, baggage, cargo, containers, conveyances, goods and postal parcels,” but based on earlier draft language of proposed IHR amendments and the WHO pandemic “accord” are likely to extend to both invasive national surveillance and mandated public health “interventions” such as vaccines and non-pharmaceutical interventions such as social distancing and lockdowns. Not to mention the continuing weaponization of public health messaging via censorship of dissenting voices and liberal use of the fear-based tactics known as information or psychological bioterrorism to mobilize public opinion in favor of WHO objectives.
The IHR amendments retain troubling language regarding censorship. These provisions have been buried in Annex 1,A.2.c., which requires State Parties to “develop, strengthen and maintain core capacities…in relation to…surveillance…and risk communication, including addressing misinformation and disinformation.”
The requirement that nations “address” “misinformation and disinformation” is fraught with opportunities for abuse. None of these terms is defined in the document. Does “addressing” it mean censoring it, and possibly punishing those who have offered divergent opinions? We have already seen how doctors and scientists who disagreed with the WHO narrative under Covid-19 were censored for their views – views that turned out to be true. Some who offered protocols not recommended by the WHO even had their licenses to practice medicine threatened or suspended. How much worse will this censorship be if it is baked in as a requirement of the International Health Regulations?
The “surveillance” requirement does not specify what is to be surveilled. The IHR amendments, however, should be read together with the proposed Pandemic Treaty, which the WHO is continuing to negotiate. Article 5 of the most recent draft of the Treaty sets forth the “One Health Approach,” which connects and balances human, animal, plant, and environmental health, giving a pretext for surveillance on all these fronts.
Meanwhile, Article 4: Pandemic Prevention and Public Health Surveillance, states:
The Parties recognize that environmental, climatic, social, anthropogenic [climate change caused by people], and economic factors increase the risk of pandemics and endeavor to identify these factors and take them into consideration in the development and implementation of relevant policies…” Through the “One Health” approach, the WHO is asserting its authority over all aspects of life on earth, all of which are apparently to be surveilled.
Regarding the IHR, Article 35 details the requirements of “Health Documents,” including those in digital format. The system of digital health documents is consistent with, and in my opinion a precursor to, the Digital IDs described by the World Economic Forum. According to the attached WEF Chart, people will need a Digital ID to:
- Access healthcare insurance and treatment
- Open bank accounts and carry out online transactions
- Travel
- Access Humanitarian Services
- Shop and conduct business transactions
- Participate in social media
- Pay taxes, vote, collect government benefits
- Own a communication device [such as a cell phone or a computer]
In other words, individuals will need Digital IDs to access almost every aspect of civilized society. All of our actions, taken with the use of Digital IDs, will be tracked and traced. If we step out of line, we can be punished by, for example, being severed from our bank accounts and credit cards – similar to what happened to the Canadian Truckers. Digital IDs are a form of mass surveillance and totalitarian control.
These Digital IDs are currently being rolled out by the World Health Organization in collaboration with the European Union. Most of us will agree that this is not the way forward to make the world safer but rather is a path leading towards a techno-totalitarian hellscape.

To support decision-making, the IHR authorizes the Director-General to appoint an “IHR Expert Roster,” an “Expert Committee” selected from the “IHR Expert Roster,” as well as a “Review Committee.” However, although the committees may make recommendations, the Director-General will have final decision authority in all relevant matters.
To further illustrate the point, the revised IHR directs that “The Director-General shall invite Member States, the United Nations and its specialized agencies and other relevant intergovernmental organizations or nongovernmental organizations in official relations with WHO to designate representatives to attend the Committee sessions. Such representatives may submit memoranda and, with the consent of the Chairperson, make statements on the subjects under discussion. They shall not have the right to vote.”
The approved amendments redefine the definition of a “Pandemic Emergency;” include a newly added emphasis on “equity and solidarity;” direct that independent Nations (“States Parties”) shall assist each other to support local production capacity for research, development, and manufacturing of health products; that equitable access to relevant health products for public health emergencies including pandemics shall be facilitated; and that developed nations shall make available “relevant terms of their research and development agreements for relevant health products related to promoting equitable access to such products during a public health emergency of international concern, including a pandemic emergency.”
The amended IHR also directs that each nation (“States Parties”) shall “develop, strengthen and maintain core capacities” for “preventing, preparing for and responding to public health risks and events,” including in relation to:
- Surveillance
- On-site Investigations
- Laboratory diagnostics, including referral of samples
- Implementation of control measures
- Access to health services and health products needed for the response
- Risk communication, including addressing misinformation and disinformation
- Logistical assistance
The amended IHR also includes copious new language, terms, and conditions relating to the responsibilities of “States Parties” to perform surveillance and transparent timely reporting of information relating to infectious disease outbreaks. This includes multiple references to information gathering, sharing, and distribution, including the need to counter the distribution of “misinformation and disinformation”.
There is the appearance that some of this new text may be informed by the recent failure of China (PRC/CCP) to provide timely and complete reporting of events and information relating to the initial SARS-CoV-2 outbreak. Unfortunately, this failure to inform in a timely manner was not unique. There is a long history of repeated, chronic problems with transparent national reporting of infectious disease outbreaks. A variety of adverse economic and political impacts are associated with infectious disease outbreaks, and this creates a strong incentive for both local politicians and public health officials to minimize initial reporting of unusual infectious disease signals or findings.
The amended IHR frequently refers to “scientific principles as well as the available scientific evidence and other relevant information” as a key factor in guiding decision-making. However, the IHR does not acknowledge the diversity of opinion surrounding what are considered sound and valid “scientific principles” or “scientific evidence,” and there is no indication that the World Health Assembly or the WHO recognizes how readily “scientific principles” and “scientific evidence” were manipulated or otherwise biased during prior public health crises, and the likelihood that this will continue to happen on a regular basis unless reforms designed to respect diversity of opinion and interpretation are implemented. There seems to be a complete lack of self-awareness of the rampant groupthink that chronically characterizes WHO decision-making during both the Covid crisis as well as prior public health events of concern.
Although many of these revisions are generally reasonable and aligned with good and practical international public health norms and actions, and in some cases are greatly improved relative to prior draft language, the recent history of WHO mismanagement and actual WHO spreading and amplification of mis- and disinformation regarding SARS-CoV-2 virology, immunology, and pathophysiology, pharmaceutical and non-pharmaceutical interventions for SARS-CoV-2 raise legitimate concerns about how these words will be interpreted and implemented.
Furthermore, the pattern of repeated arbitrary, capricious, and scientifically unjustifiable decisions regarding Covid and monkeypox suggests that expanding the authority of either the Director-General or the WHO is unwise at this time. Rather, more mature, thoughtful, and prudent evaluation of that recent experience argues for reduced rather than expanded authority, and for a more decentralized, multilateral model for the management of global and regional public health risks and events. The world does not need more condescending authoritarianism from those entrusted to facilitate international cooperation in public health.
Just speaking in terms of best practices, it is clearly inappropriate to rely on administrators with such a vested personal interest in the outcome to be so intimately involved in crafting sweeping international policy changes. This revision process should have been managed by an independent commission of seasoned, objective experts who were carefully vetted to minimize potential conflict of interest.
The hasty willingness to bypass its own charter by unilaterally and arbitrarily jamming these changes through on extremely short notice raises further concerns regarding the reliability, maturity, and competency of the WHO, the World Health Assembly, and the Director-General to provide the calm, steady hand so sorely needed after the mismanaged major public health catastrophe and global trauma which all have experienced over the last four years.
The world, its inhabitants, those who work to provide medical care, and the overall world health enterprise deserve better.
Specific clauses of concern in the revised International Health Regulations include the following:
PART I – DEFINITIONS, PURPOSE AND SCOPE, PRINCIPLES AND RESPONSIBLE AUTHORITIES
Article 1 Definitions
“National IHR Authority” means the entity designated or established by the State Party at the national level to coordinate the implementation of these Regulations within the jurisdiction of the State Party;
“National IHR Focal Point” means the national centre, designated by each State Party, which shall be accessible at all times for communications with WHO IHR Contact Points under these Regulations;
“pandemic emergency” means a public health emergency of international concern that is caused by a communicable disease and:
- (i) has, or is at high risk of having, wide geographical spread to and within multiple States; and
- (ii) is exceeding, or is at high risk of exceeding, the capacity of health systems to respond in those States; and
- (iii) is causing, or is at high risk of causing, substantial social and/or economic disruption, including disruption to international traffic and trade; and
- (iv) requires rapid, equitable, and enhanced coordinated international action, with whole-of-government and whole-of-society approaches.
“public health emergency of international concern” means an extraordinary event which is determined, as provided in these Regulations:
- (i) to constitute a public health risk to other States through the international spread of disease; and
- (ii) to potentially require a coordinated international response;
“relevant health products” means those health products needed to respond to public health emergencies of international concern, including pandemic emergencies, which may include medicines, vaccines, diagnostics, medical devices, vector control products, personal protective equipment, decontamination products, assistive products, antidotes, cell- and gene-based therapies, and other health technologies;
“temporary recommendation” means non-binding advice issued by WHO pursuant to Article 15 for application on a time-limited, risk-specific basis, in response to a public health emergency of international concern, so as to prevent or reduce the international spread of disease and minimize interference with international traffic;
Article 2 Purpose and scope
The purpose and scope of these Regulations are to prevent, prepare for, protect against, control, and provide a public health response to the international spread of disease in ways that are commensurate with and restricted to public health risk and which avoid unnecessary interference with international traffic and trade.
Article 3 Principles
1. The implementation of these Regulations shall be with full respect for the dignity, human rights, and fundamental freedoms of persons, and shall promote equity and solidarity.
2. The implementation of these Regulations shall be guided by the Charter of the United Nations and the Constitution of the World Health Organization.
3. The implementation of these Regulations shall be guided by the goal of their universal application for the protection of all people of the world from the international spread of disease.
4. States have, in accordance with the Charter of the United Nations and the principles of international law, the sovereign right to legislate and to implement legislation in pursuance of their health policies. In doing so, they should uphold the purpose of these Regulations.
Article 4 Responsible authorities
1. Each State Party shall designate or establish, in accordance with its national law and context, one or two entities to serve as National IHR Authority and a National IHR Focal Point, as well as the authorities responsible within its respective jurisdiction for the implementation of health measures under these Regulations.
The National IHR Authority shall coordinate the implementation of these Regulations within the jurisdiction of the State Party.
PART II – INFORMATION AND PUBLIC HEALTH RESPONSE
Article 5 Surveillance
Each State Party shall develop, strengthen and maintain, as soon as possible but no later than five years from the entry into force of these Regulations for that State Party, the core capabilities to prevent, detect, assess, notify and report events in accordance with these Regulations,
WHO shall collect information regarding events through its surveillance activities and assess their potential to cause international disease spread and possible interference with international traffic. Information received by WHO under this paragraph shall be handled in accordance with Articles 11 and 45 where appropriate.
Article 7 Information-sharing during unexpected or unusual public health events
If a State Party has evidence of an unexpected or unusual public health event within its territory, irrespective of origin or source, which may constitute a public health emergency of international concern, it shall provide to WHO all relevant public health information. In such a case, the provisions of Article 6 shall apply in full.
In determining whether an event constitutes a public health emergency of international concern, including, when appropriate, a pandemic emergency, the Director-General shall consider:
(a) information provided by the State(s) Party(ies);
(b) the decision instrument contained in Annex 2;
(c) the advice of the Emergency Committee;
(d) scientific principles as well as the available scientific evidence and other relevant information; and
(e) an assessment of the risk to human health, of the risk of international spread of disease and of the risk of interference with international traffic.
If the Director-General determines that an event constitutes a public health emergency of international concern, the Director-General shall further determine, having considered the matters contained in paragraph 4, whether the public health emergency of international concern also constitutes a pandemic emergency.
Article 13 Public health response, including equitable access to relevant health products
WHO shall facilitate, and work to remove barriers to, timely and equitable access by States Parties to relevant health products after the determination of and during a public health emergency of international concern, including a pandemic emergency, based on public health risks and needs. To that effect, the Director-General shall:
support States Parties, upon their request, in scaling up and geographically diversifying the production of relevant health products, as appropriate, through relevant WHO-coordinated and other networks and mechanisms, subject to Article 2 of these Regulations, and in accordance with relevant international law;
- share with a State Party, upon its request, the product dossier related to a specific relevant health product, as provided to WHO by the manufacturer for approval and where the manufacturer has consented, within 30 days of receiving such request, for the purpose of facilitating regulatory evaluation and authorization by the State Party.; and
- support States Parties, upon their request, and, as appropriate, through relevant WHO-coordinated and other networks and mechanisms, pursuant to subparagraph 8(c) of this Article, to promote research and development and strengthen local production of quality, safe and effective relevant health products, and facilitate other measures relevant for the full implementation of this provision.
Pursuant to paragraph 5 of this Article and paragraph 1 of Article 44 of these Regulations, and upon request of other States Parties or WHO, States Parties shall undertake, subject to applicable law and available resources, to collaborate with, and assist each other and to support WHO-coordinated response activities, including through:
- engaging with and encouraging relevant stakeholders operating in their respective jurisdictions to facilitate equitable access to relevant health products for responding to a public health emergency of international concern, including a pandemic emergency; and
- making available, as appropriate, relevant terms of their research and development agreements for relevant health products related to promoting equitable access to such products during a public health emergency of international concern, including a pandemic emergency.
PART III – RECOMMENDATIONS
Article 15 Temporary recommendations
If it has been determined in accordance with Article 12 that a public health emergency of international concern, including a pandemic emergency, is occurring, the Director-General shall issue temporary recommendations in accordance with the procedure set out in Article 49. Such temporary recommendations may be modified or extended as appropriate, including after it has been determined that a public health emergency of international concern, including a pandemic emergency, has ended, at which time other temporary recommendations may be issued as necessary for the purpose of preventing or promptly detecting its recurrence.
Temporary recommendations may include health measures to be implemented by the State(s) Party(ies) experiencing the public health emergency of international concern, including a pandemic emergency, or by other States Parties, regarding persons, baggage, cargo, containers, conveyances, goods, including relevant health products, and/or postal parcels to prevent or reduce the international spread of disease and avoid unnecessary interference with international traffic.
The Director-General, when communicating to States Parties the issuance, modification or extension of temporary recommendations, should provide available information on any WHO-coordinated mechanism(s) concerning access to, and allocation of, relevant health products, as well as on any other allocation and distribution mechanisms and networks.
Temporary recommendations may be terminated in accordance with the procedure set out in Article 49 at any time and shall automatically expire three months after their issuance. They may be modified or extended for additional periods of up to three months. Temporary recommendations may not continue beyond the second World Health Assembly after the determination of the public health emergency of international concern, including a pandemic emergency, to which they relate.
Article 16 Standing recommendations
The Director-General, when communicating to States Parties the issuance, modification or extension of standing recommendations, should provide available information on any WHO-coordinated mechanism(s) concerning access to, and allocation of, relevant health products as well as on any other allocation and distribution mechanisms and networks.
Article 18 Recommendations with respect to persons, baggage, cargo, containers, conveyances, goods and postal parcels
Recommendations issued by WHO to States Parties with respect to persons may include the following advice:
- no specific health measures are advised;
- review travel history in affected areas;
- review proof of medical examination and any laboratory analysis;
- require medical examinations;
- review proof of vaccination or other prophylaxis;
- require vaccination or other prophylaxis;
- place suspect persons under public health observation;
- implement quarantine or other health measures for suspect persons;
- implement isolation and treatment where necessary of affected persons;
- implement tracing of contacts of suspect or affected persons;
- refuse entry of suspect and affected persons;
- refuse entry of unaffected persons to affected areas;
- implement exit screening and/or restrictions on persons from affected areas.
Recommendations issued by WHO to State Parties shall, as appropriate, take into account the need to:
(a) facilitate international travel, particularly of health and care workers and persons in life-threatening or humanitarian situations. This provision is without prejudice to Article 23 of these Regulations; and
(b) maintain international supply chains, including for relevant health products and food supplies.
PART V – PUBLIC HEALTH MEASURES
Chapter I – General provisions
Article 23 Health measures on arrival and departure
On the basis of evidence of a public health risk obtained through the measures provided in paragraph 1 of this Article, or through other means, States Parties may apply additional health measures, in accordance with these Regulations, in particular, with regard to a suspect or affected traveller, on a case-by-case basis, the least intrusive and invasive medical examination that would achieve the public health objective of preventing the international spread of disease.
No medical examination, vaccination, prophylaxis or health measure under these Regulations shall be carried out on travellers without their prior express informed consent or that of their parents or guardians, except as provided in paragraph 2 of Article 31, and in accordance with the law and international obligations of the State Party.
Travellers to be vaccinated or offered prophylaxis pursuant to these Regulations, or their parents or guardians, shall be informed of any risk associated with vaccination or with non-vaccination and with the use or non-use of prophylaxis, in accordance with the law and international obligations of the State Party. States Parties shall inform medical practitioners of these requirements in accordance with the law of the State Party.
Any medical examination, medical procedure, vaccination or other prophylaxis which involves a risk of disease transmission shall only be performed on, or administered to, a traveller in accordance with established national or international safety guidelines and standards so as to minimize such a risk.
Chapter III – Special provisions for travellers
Article 31 Health measures relating to entry of travellers
Invasive medical examination, vaccination or other prophylaxis shall not be required as a condition of entry of any traveller to the territory of a State Party, except that, subject to Articles 32, 42 and 45, these Regulations do not preclude States Parties from requiring medical examination, vaccination or other prophylaxis or proof of vaccination or other prophylaxis:
- when necessary to determine whether a public health risk exists;
- as a condition of entry for any travellers seeking temporary or permanent residence;
- as a condition of entry for any travellers pursuant to Article 43 or Annexes 6 and 7; or
- which may be carried out pursuant to Article 23.
2. If a traveller for whom a State Party may require a medical examination, vaccination or other prophylaxis under paragraph 1 of this Article fails to consent to any such measure, or refuses to provide the information or the documents referred to in paragraph 1(a) of Article 23, the State Party concerned may, subject to Articles 32, 42 and 45, deny entry to that traveller. If there is evidence of an imminent public health risk, the State Party may, in accordance with its national law and to the extent necessary to control such a risk, compel the traveller to undergo or advise the traveller, pursuant to paragraph 3 of Article 23, to undergo:
- the least invasive and intrusive medical examination that would achieve the public health objective;
- vaccination or other prophylaxis; or
- additional established health measures that prevent or control the spread of disease, including isolation, quarantine or placing the traveller under public health observation.
PART VI – HEALTH DOCUMENTS
Article 35 General rule
No health documents, other than those provided for under these Regulations or in recommendations issued by WHO, shall be required in international traffic, provided however that this Article shall not apply to travellers seeking temporary or permanent residence, nor shall it apply to document requirements concerning the public health status of goods or cargo in international trade pursuant to applicable international agreements. The competent authority may request travellers to complete contact information forms and questionnaires on the health of travellers, provided that they meet the requirements set out in Article 23.
Health documents under these Regulations may be issued in non-digital format or digital format, subject to the obligations of any State Party regarding the format of such documents deriving from other international agreements.
Regardless of the format in which health documents under these Regulations have been issued, said health documents shall conform to the Annexes, referred to in Articles 36 to 39, as applicable, and their authenticity shall be ascertainable.
WHO, in consultation with States Parties, shall develop and update, as necessary, technical guidance, including specifications or standards related to the issuance and ascertainment of authenticity of health documents, both in digital format and non-digital format. Such specifications or standards shall be in accordance with Article 45 regarding treatment of personal data.
PART VIII – GENERAL PROVISIONS
Article 43 Additional health measures
In determining whether to implement the health measures referred to in paragraph 1 of this Article or additional health measures under paragraph 2 of Article 23, paragraph 1 of Article 27, paragraph 2 of Article 28 and paragraph 2(c) of Article 31, States Parties shall base their determinations upon:
- scientific principles;
- available scientific evidence of a risk to human health, or where such evidence is insufficient, the available information, including from WHO and other relevant intergovernmental organizations and international bodies; and
- any available specific guidance or advice from WHO.
A State Party implementing additional health measures referred to in paragraph 1 of this Article which significantly interfere with international traffic shall provide to WHO the public health rationale and relevant scientific information for it. WHO shall share this information with other States Parties and shall share information regarding the health measures implemented. For the purpose of this Article, significant interference generally means refusal of entry or departure of international travellers, baggage, cargo, containers, conveyances, goods, and the like, or their delay, for more than 24 hours.
Article 44 Collaboration and, assistance and financing
States Parties shall undertake to collaborate with each other, to the extent possible in:
- the detection and assessment of, preparedness for, and response to, events as provided under these Regulations;
- the provision or facilitation of technical cooperation and logistical support, particularly in the development, strengthening and maintenance of the public health core capacities required under Annex 1 of these Regulations;
- the mobilization of financial resources, including through relevant sources and funding mechanisms to facilitate implementation of their obligations under these Regulations in particular to address the needs of developing countries
States Parties, subject to applicable law and available resources, shall maintain or increase domestic funding, as necessary, and collaborate, including through international cooperation and assistance, as appropriate, to strengthen sustainable financing to support the implementation of these Regulations.
States Parties shall undertake to collaborate, to the extent possible, to:
- encourage governance and operating models of existing financing entities and funding mechanisms to be regionally representative and responsive to the needs and national priorities of developing countries in the implementation of these Regulations;
- identify and enable access to financial resources, including through the Coordinating Financial Mechanism, established pursuant to Article 44bis, necessary to equitably address the needs and priorities of developing countries, including for developing, strengthening and maintaining core capacities.
Article 44bis – Coordinating Financial Mechanism
In support of the objectives set out in Paragraph 1 of this Article, the Mechanism shall:
- use or conduct relevant needs and funding gap analyses;
- promote harmonization, coherence and coordination of existing financing instruments;
- identify all sources of financing that are available for implementation support and make this information available to States Parties;
- provide advice and support, upon request, to States Parties in identifying and applying for financial resources for strengthening core capacities, including those relevant for pandemic emergencies;
- leverage voluntary monetary contributions for organizations and other entities supporting States Parties to develop, strengthen and maintain their core capacities, including those relevant for pandemic emergencies.
Article 45 Treatment of personal data
Health information collected or received by a State Party pursuant to these Regulations from another State Party or from WHO which refers to an identified or identifiable person shall be kept confidential and processed anonymously, as required by national law.
Notwithstanding paragraph 1, States Parties may process and disclose and process personal data where essential for the purposes of assessing and managing a public health risk, but State Parties, in accordance with national law, and WHO must ensure that the personal data are:
- processed fairly and lawfully, and not further processed in a way incompatible with that purpose;
- adequate, relevant and not excessive in relation to that purpose;
- accurate and, where necessary, kept up to date; every reasonable step must be taken to ensure that data which are inaccurate or incomplete are erased or rectified; and
- not kept longer than necessary.
Upon request, WHO shall as far as practicable provide an individual with his or her personal data referred to in this Article in an intelligible form, without undue delay or expense and, when necessary, allow for correction.
ANNEX 1: CORE CAPACITIES
States Parties shall utilize existing national structures and resources to meet their core capacities requirements under these Regulations, including with regard to:
- their prevention surveillance, reporting, notification, verification, preparedness, response and collaboration activities; and
- their activities concerning designated airports, ports and ground crossings.
A. CORE CAPACITIES REQUIREMENTS FOR PREVENTION, SURVEILLANCE, PREPAREDNESS AND RESPONSE
At the local community level and/or primary public health response level (hereinafter the “Local level”), each State Party shall develop, strengthen and maintain the core capacities:
to detect events involving disease or death above expected levels for the particular time and place in all areas within the territory of the State Party; and
to report all available essential information immediately to the appropriate level of health- care response. At the community level, reporting shall be to local community health-care institutions or the appropriate health personnel. At the primary public health response level, reporting shall be to the intermediate or national response level, depending on organizational structures. For the purposes of this Annex, essential information includes the following: clinical descriptions, laboratory results, sources and type of risk, numbers of human cases and deaths, conditions affecting the spread of the disease and the health measures employed; and
- to prepare for the implementation of, and implement immediately, preliminary control measures immediately.;
- to prepare for the provision of, and facilitate access to health services necessary for responding to public health risks and events; and
- to engage relevant stakeholders, including communities, in preparing for and responding to public health risks and events.
At the intermediate public health response levels (hereinafter the “Intermediate level”), where applicable, each State Party shall develop, strengthen and maintain the core capacities:
At the intermediate public health response levels (hereinafter the “Intermediate level”), where applicable,1, each State Party shall develop, strengthen and maintain Tthe core capacities:
(a) to confirm the status of reported events and to support or implement additional control measures; and
(b) to assess reported events immediately and, if found urgent, to report all essential information to the national level. For the purposes of this Annex, the criteria for urgent events include serious public health impact and/or unusual or unexpected nature with high potential for spread.; and
to coordinate with and support the Local level in preventing, preparing for and responding to public health risks and events, including in relation to:
- surveillance;
- on-site investigations;
- laboratory diagnostics, including referral of samples;
- implementation of control measures;
- access to health services and health products needed for the response;
- risk communication, including addressing misinformation and disinformation;
- logistical assistance (e.g. equipment, medical and other relevant supplies and transport).
At the national level, Each State Party shall develop, strengthen and maintain the core capacities:
- to assess all reports of urgent events within 48 hours; and
- to notify WHO immediately through the National IHR Focal Point when the assessment indicates the event is notifiable pursuant to paragraph 1 of Article 6 and Annex 2 and to inform WHO as required pursuant to Article 7 and paragraph 2 of Article 9.
Public health prevention, preparedness and response.
Each State Party shall develop, strengthen and maintain the core capacities for:
- rapidly determining rapidly the control measures required to prevent domestic and international spread;
- surveillance;
- deploying specialized staff,
- laboratory analysis of samples (domestically or through collaborating centres) and;
- logistical assistance (e.g. equipment, medical and other relevant supplies and transport);
- providing on-site assistance as required to supplement local investigations;
- developing and/or disseminating guidance for clinical case management and infection prevention and control;
- access to health services and health products needed for the response;
- risk communication, including addressing misinformation and disinformation;
- providing a direct operational link with senior health and other officials to approve rapidly and implement containment and control measures;
- providing direct liaison with other relevant government ministries;
- providing, by the most efficient means of communication available links with hospitals, clinics, airports, ports, ground crossings, laboratories and other key operational areas for the dissemination of information and recommendations received from WHO regarding events in the State Party’s own territory and in the territories of other States Parties;
- establishing, operating and maintaining a national public health emergency response plan, including the creation of multidisciplinary/multisectoral teams to respond to events that may constitute a public health emergency of international concern;
ANNEX 2: DECISION INSTRUMENT FOR THE ASSESSMENT AND NOTIFICATION OF EVENTS THAT MAY CONSTITUTE A PUBLIC HEALTH EMERGENCY OF INTERNATIONAL CONCERN
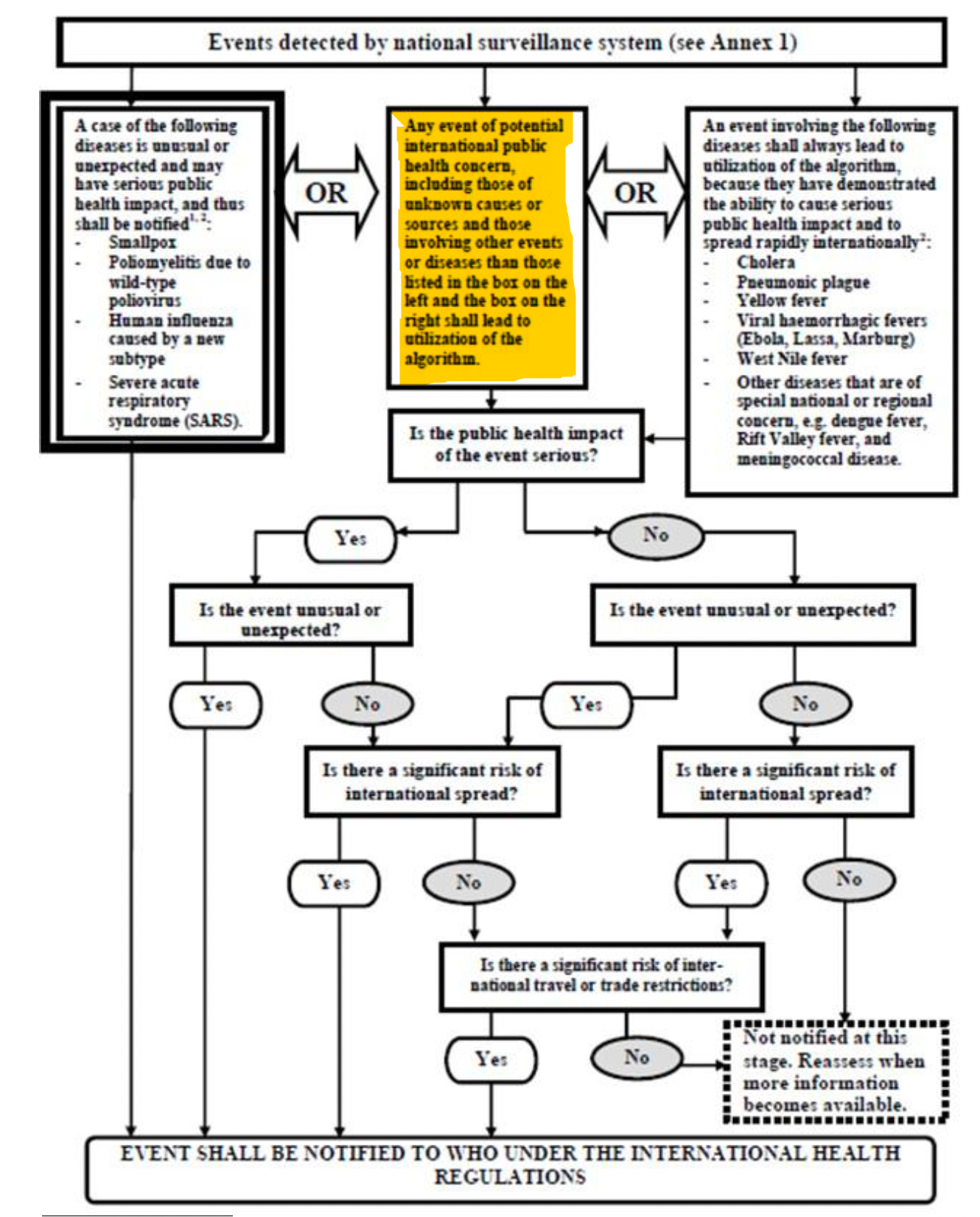
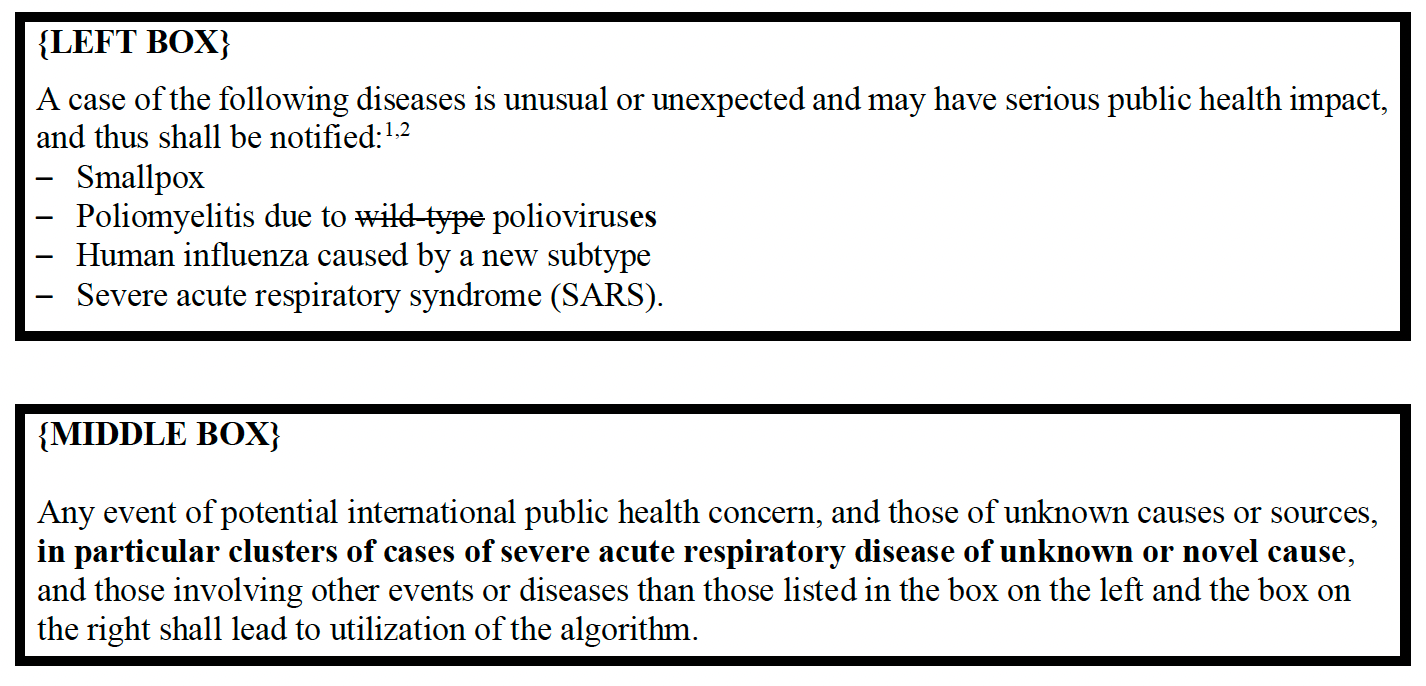

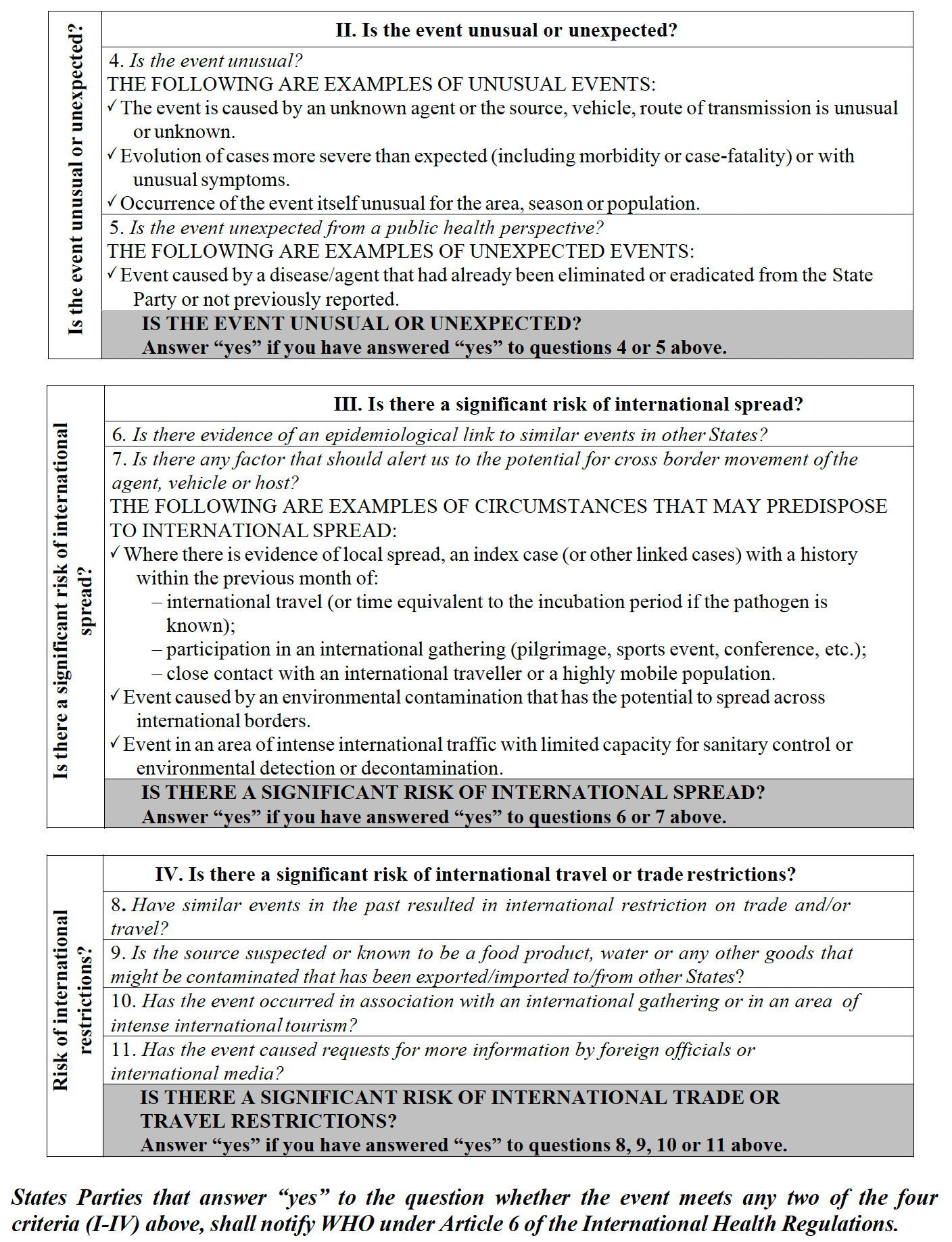 Republished from the author’s Substack
Republished from the author’s Substack
Published under a Creative Commons Attribution 4.0 International License
For reprints, please set the canonical link back to the original Brownstone Institute Article and Author.


No comments:
Post a Comment