Trump’s 63 Million Doses of Hydroxychloroquine Could Have Been Great for America
Following Trump’s proposal, HCQ suddenly came under an unwarranted full-scale attack from federal officials, the press, so-called “fact-checkers,” and university professors. Many of the attacks contained outright falsehoods about HCQ’s pharmacology and safety or Trump’s endeavor to make HCQ available to eligible patients.
The FDA initially issued an emergency use authorization (EUA) for HCQ in March 2020, but withdrew authorization on June 15th 2020, stating the drug is “unlikely to be effective in treating COVID-19 for the [EUA] authorized uses.” Around the same time, the FDA also wrote a methodologically questionable report criticizing HCQ’s safety. The FDA’s narrative was based on preliminary and time-compartmentalized findings, and not a reflection of historical safety or based on the appropriate clinical use of HCQ dosing, prescribing, timing, and duration. The FDA then seemed to label its findings as conclusive, figuratively slamming the door shut on the consideration of new findings.
FDA Hydroxychloroquine Safety Assessment Based on: Known Overdoses, and Clinically Unsupervised Uses
According to its uncharacteristically brief 15-page safety review memorandum of HCQ published on May 19, 2020, the FDA considered data from the National Poison Data System (NPDS) which means it seems to have included the use of non-pharmaceutical-grade and/or self-administered HCQ and/or overdose data in its clinical evaluation.
Obviously, overdose or any non-medically supervised, self-diagnosis, and/or self-dosing of any prescription drug has a higher potential to result in adverse events, especially because the antiviral dose/duration of HCQ and/or chloroquine were not immediately obvious to the lay public. Of note, “if it bleeds it leads”-type sensationalized news reporting caused anxious and desperate Americans with incomplete or incorrect transmission and mortality information to go so far as to self-administer “fish tank” chloroquine-containing cleaning products of containing other chemicals to treat themselves from possible Covid-19 exposure. This led to severe illness due to the ingestion of toxic ingestions or overdoses, sometimes up to the point of death.
Heavy Reliance on the FDA’s Adverse Event Reporting System Database
In its report, the FDA also referenced its Adverse Event Reporting System (FAERS) which according to Table 6 only contained around 256 applicable adverse event reports over a five-month period in the entire world when administered for Covid-19. Of note, most of the reports cited by the FDA included doses which were either unknown and/or were anywhere from 2x to 6x the recommended maximum maintenance dose of HCQ for any clinical indication per FDA and manufacturer’s dosing recommendations. On top of that, it appears that the FDA gave no consideration to HCQ base/salt formulations or critical weight-based dosing of HCQ (on a mg/kg basis) as it did not appear to investigate or report patient weights. It is unknown if any organ function assessments were considered as part of their safety collection or evaluation – an important consideration since HCQ is hepatically metabolized and renally excreted, with dosage adjustments potentially needed for out-of-range liver or kidney function.
These and other important mitigating factors would have been needed for a suitable evaluation of HCQ safety by the FDA.
FDA Seemed to Include Well-Known Drug-Drug Interactions and Unknown Quality Likely from Rogue Internet Pharmacies:
Well- established drug-drug interactions, some established as far back as the 1980s, occurred in individuals who obtained HCQ by circumventing normal patient/prescribing channels. In addition to the incorrect/excessive dose/durations used, the FDA’s report details how HCQ appeared to have been dispensed from rogue overseas pharmacies which have a history of producing consumer drugs with poor quality and toxic contaminants.
In its report, the FDA showed that the majority of all adverse event cases (69% of the 331 total HCQ safety reports) involved males with a median age in the early 60s, yet the FDA used those negative findings to recommend against the use of HCQ in every age group.
On top of that, Page 7 of the report showed most of the 109 serious HCQ cardiac adverse event cases cited by the FDA also directly contraindicated well-established FDA and/or manufacturer dosing guidelines for HCQ.
Specifically:
- 92 of the 109 serious cardiac cases (84%) reported concomitant use of at least one other medication that prolongs the QT interval.
- 75 (69%) of cardiac cases specifically reported concomitant use of azithromycin.
- 22/25 [88%] of the fatal cases reported use of a concomitant QT-prolonging medication.
In other words, the FDA included well-known, clinically unsound, long-established, drug-drug interactions, contraindicated uses, and/or prescribing errors in denigrating HCQ’s safety.
It is fair to say that 84% of cardiac adverse events (and possibly the majority of other reported HCQ adverse events listed in the FDA’s memorandum) could have been prevented by clinical patient education, appropriate clinical supervision, and the appropriate pharmacist dispensing/counseling of a medication of known quality from a US pharmacy, including cardiac assessments and the appropriate checks for drug-drug interactions. Even more adverse events could have likely been prevented with a brief cardiac history evaluation and/or an electrocardiogram. In fact, Lopinavir/ritonavir (Paxlovid), used to treat Covid, has an established QT prolongation effect like HCQ, but since warnings about drug-drug interactions are well-publicized and Paxlovid is dispensed under physician/pharmacist supervision, reported cardiac adverse events are uncommon, avoiding cardiac and other safety concerns.
No one drug is ever appropriate for every single person, and not everyone is eligible to be dosed with HCQ for underlying reasons including organ function, cardiac conditions, and/or the risk for established adverse events. However, drug-drug interactions and adverse events can be mitigated by dose adjustment and/or temporarily pausing other pharmacotherapies for the limited duration of time that HCQ is needed to prevent or treat early Covid-19 post-exposure. Separately from that, other FDA admonishments regarding safety described in its report appear to instead describe those related to long-term dosing not needed for Covid-19 early- or pre-exposure indications or temporary and widely touted “stop the spread” initiatives.
Hydroxychloroquine Is Objectively Safe When Used Appropriately:
Following the unauthorized pandemic surge of HCQ use, a clearer message from America’s FDA to the American public about the safe uses of HCQ should have taken place but didn’t. Instead, the FDA kept silent, did not specifically warn consumers about circumventing America’s medical and pharmacy system, and let Americans make (sometimes fatal) HCQ-related usage errors. The FDA then released a surveillance memorandum essentially pronouncing HCQ as “unsafe” for Covid-19. The FDA made that safety declaration despite the CDC promoting the use of HCQ back in 2019 as safe and “a relatively well tolerated medicine.”
In fact, HCQ is considered so safe for non-Covid-19 indications that the CDC states that “HCQ can be prescribed to adults and children of all ages. It can also be safely taken by pregnant women and nursing mothers.” The CDC was referencing the long-term use of HCQ for chronic disease treatments.
If the CDC considers it safe for long-term treatment, it is only logical to assume that it would certainly be safe for short-term use against quickly spreading viral infections like Covid-19.
Hundreds of other studies (listed in the bibliography of this paper) have been shown to only rarely report safety concerns during limited duration of HCQ administered for Covid-19. Of those, almost all were minor, and of those, all appeared to resolve upon drug discontinuation. The specific reports of HCQ safety are detailed in the summaries following each citation in the bibliography.
FDA Duplicity: FAERS Is OK to Vilify Hydroxychloroquine Safety, but NOT OK to Denigrate mRNA Injection Safety:
The FDA’s heavy reliance on AERS reports in denigrating HCQ in its report was not only biased – it was ironic.
In the past, the FDA and the NIH have repeatedly scolded the use of FAERS as having “not been verified” and “not establish[ing] causation” and that “correlation is not causation” as an excuse to seemingly selectively ignore correlation, and how FAERS “Rates of occurrence cannot be established with [FAERS] reports” and how FAERS findings have “no definitive proof of the causal relationship between exposure to the product and the reported event.”
Therefore, by the FDA’s own account, any of the supposedly valid 256 HCQ FAERS cases cited in its reports could have been:
1) Exaggerated,
2) Not allowed to be used to calculate or imply an incidence rate, (which the press did anyway),
3) Attributable to causes other than HCQ and
4) *If* used to calculate rate, the potential calculated incidence rate of 291 cases worldwide would represent an exceptionally small relative adverse event incidence, but we don’t know that because the FDA did not provide or estimate the 291 cases versus the total number of patients dosed. In other words, the 291 figure is the numerator, but what is the denominator?
The FDA based its safety decision on a worldwide solicitation of a total of 331 reports from all sources (a huge fraction of which were obvious clinically inappropriate uses and/or overdoses and/or in people over the age of 60), over an approximate five-month period, including 256 worldwide reports in FAERS, including: 25 total reports in the entirety of published medical literature, 20 reports form the NPDS, and 11 “other” reports of nebulous origin. One-hundred and nine total HCQ/chloroquine were adjudicated as “serious cardiac related” and an additional 113 were “serious non-cardiac.”
No indication was given that non-fatal adverse events didn’t fully resolve following short-term HCQ use discontinuation or the full course of treatment to prevent or treat Covid-19.
As is clear, the adverse events that occurred had mitigating factors. HCQ is considered to be a safe drug with relatively few adverse event reports. In fact, a comprehensive search for the total number of adverse event reports for HCQ/chloroquine over the past 55 years of worldwide use (including very minor adverse events and those adverse events originating from known drug-drug interactions) in the FDA AERS database showed a grand total of 32,011 cases according to the most recent database update in 2024. The clinical use of chemical precursors of HCQ date back nearly 100 years, but safety and adverse event collection databases only date back to around 1969.
Relative Safety of Hydroxychloroquine’s 32,011 vs >1,000,000 mRNA FDA Reports:
While 32,011 adverse event reports is not inconsequential, compare that to the over 1 million adverse event reports submitted to the FDA’s Vaccine Adverse Event Reporting System (VAERS) for mRNA Covid-19 shots, total, just since 2021 (i.e. ~3.5 years) – and not in the whole world – but in the United States alone, with tens of thousands of those million known to be serious, permanent, and/or deadly.
In fact, the number of worldwide deaths reported from mRNA injections (over 37,500) during its three years on the market exceeds the total number of reported adverse events that occurred during the entire 55-year history of HCQ use. Of note, an abundance of HCQ adverse events associated with the short-term use of HCQ were minor, and included things like nausea, decreased appetite, and fatigue, which are adverse events associated with many different drugs.
Despite that, around the same time that the deluge of mRNA adverse events were reported, America’s self-proclaimed “expert fact-checkers” repeatedly used their megaphones to scold Americans that HCQ was “unsafe.” Major medical research centers and fact-checkers told Americans that numerous mRNA adverse event reports and unexplained sudden deaths and clinical reports of cancers were “not causation” and on top of that, Covid mRNA shots are additionally “Proven Safe” and “Not Dangerous” and “Do NOT Need To Be Withdrawn From The Market” [capitalization theirs].
One does not need to be an expert in drug safety epidemiology to distinguish the incongruity between the continued EUA followed by full approval for novel mRNA Covid injections with hundreds of thousands of adverse events, compared to rapid HCQ EUA withdrawal following 331 worldwide HCQ adverse event reports, an abundance of seemingly related to improper sourcing/use/dosing/supervision.
Major Journal Articles Declaring Hydroxychloroquine as Unsafe for Covid-19:
Prior to HCQ’s EUA removal, a seemingly highly coordinated and unquestionably harmonized message came out against HCQ from American’s press, making it appear that Trump’s HCQ recommendation was not only “unsafe” but that it also “didn’t work” for Covid-19. Harvard, Stanford, and Scripps Institute scientists (respectively) warned Americans via a Washington Post article that Trump’s efforts to employ HCQ were “desperate” and “If there was ever hope for [HCQ], this is the death of it” and “It’s one thing not to have benefit, but [HCQ] shows distinct harm.”
The scientists quoted above were referencing Lancet and New England Journal of Medicine articles that were widely referenced as a means to criticize Trump’s proposal for implementation of HCQ for Covid-19. Both publications were later retracted by journal editors due to being fraudulent.
They were retracted by the journals when its authors “refused to give [auditors] access to all the data they asked for” following publication when results were questioned by outside scientists who questioned why HCQ, with such a historical safety record suddenly appeared so unsafe for Covid-19 patients. Questions led to an investigation which eventually revealed that none of the publication’s authors nor journal “peer-reviewers” had likely ever seen the 96,032 patient data in the first place, because it never existed. The critical question is: why did those so-called “peer-reviewers” permit publication of highly incongruent safety findings for HCQ before thoroughly confirming those findings?
Following the redaction, Lancet’s editor, Richard Horton, stated he was appalled with the authors, calling the HCQ-lambasting study “a shocking example of research misconduct in the middle of a global health emergency.” Lancet’s editor did “…apologise to the editors and to readers of the Lancet for the difficulties that this has caused.”
The same press that had no reservations about hysterically labeling the Trump administration as wrong for attempting to advance HCQ and coordinating messaging against him was almost completely silent and of course did not coordinate or harmonize any correction admitting that they had not verified highly questionable data even though people’s lives were at stake.
It is known today that the press and medical journals were not just wrong but outrageously wrong, about both their declaration and the methodology they employed to arrive at their conclusions despite supposedly delivering “real truth,” being “fact-checked” and delivering “real facts,” and/or claiming to be “peer-reviewed research.”
Consequences for Publishing False Hydroxychloroquine Data?
The now retracted articles had been primarily authored by Mandeep Mehra MD, a Harvard Medical School professor who also serves as the director of the Brigham and Women’s Hospital Heart and Vascular Center. On a side note, Dr. Mehra retains both positions/titles to this very day, in what has become an all too familiar pattern of Harvard and other prominent university officials maintaining their prestigious and lucrative positions despite publication fraud and/or incompetence.
The second author on both papers was Sapan Desai MD, who had claimed to have the world’s largest and most sophisticated patient databases, under his now defunct Illinois-based company, Surgisphere. As it turns out, his database which had reported harmful effects tied to HCQ among patients with Covid-19 never existed, and the “peer-reviewers” at the supposedly “top tier” Lancet, and New England Journal of Medicine whose job it was to critically review the data never actually verified any of the highly questionable epidemiology findings contrasting HCQ’s legendary safety record in autoimmune disorders and malaria, as detailed in an abundance of publications plus FDA’s AERS database.
The third author of the paper, Professor Frank Ruschitzka MD, (like Harvard’s Mehra) still holds his Chairmanship of the University Heart Center and the Department of Cardiology at the University Hospital in Zürich, Switzerland.
The fourth author on the paper, Amit Patel MD is related to Sapan Desai through marriage. He is the only author who has been directly “punished” for publishing fraudulent data, having “mutually agreed” to having had his unpaid, adjunct faculty position at the University of Utah, terminated.
Further investigations into Sapan Desai by others found multiple, egregious incidences of medical fraud along with multiple incidence of clinical malpractice and negligence preceding his Lancet publication — something that journal peer reviews should have been tuned in to and considered while reviewing his data for publication.
Although Dr. Desai was allowed to voluntarily surrender his medical licenses in Ohio and Illinois, it appeared to be related to patient care-related matters. An internet search did not show any pending litigation against Dr. Desai for fraudulently publishing medical data. It is unclear if Dr. Desai or any other authors are facing criminal charges for falsifying negative clinical findings about HCQ.
It is unknown what if any ramifications occurred to either journals’ “peer-reviewers” as a result of allowing these fraudulent safety-incongruent HCQ data to be published.
Blind Regurgitation of Faulty Journal Publications by the Lay Press:
Politicians and “trusted journalists” with zero education or training in science – let alone no background in pharmacology – let alone no background in the intricacies of investigational medicine, epidemiology, or the clinical assessment of drug safety – exuberantly leapt to criticize Trump’s HCQ proposal, based on both unverified or fraudulently written journal publications.
Here are just some of the many quotes:
- The New York Times stated that Trump’s HCQ efforts were “Likely for nothing” and gushed about how “Medical experts across the country… applauded the FDA’s withdrawal of the [emergency use] waiver” in referring to HCQ.
- Another Washington Post article stated that HCQ use for Covid-19 “makes no sense” and “no medical evidence supports Trump’s hydroxychloroquine obsession” in a piece titled: “…People who take his [Trump’s] advice may die” in referring to HCQ.
- ABC News stated that Trump’s recommended use of HCQ for Covid-19 “directly contradicted guidance from the nation’s top public health agencies and officials.”
- The medical and pharmacology heavyweights at Salon.com opined that HCQ was “Revealed to be useless for treating COVID-19” stating that Trump’s “[HCQ] stockpile epitomizes presidential incompetence.”
- Arizona Democratic Representative Raul Grijalva adjudicated HCQ as “useless” stating that Trump used “lies and falsehoods” promoting HCQ, seething that “Trump creates a crisis everywhere he goes and consistently puts his own desire to be right above the health needs of everyday Americans.”
- The reliably gormless Arizona Republic journalist E.J. Montini, who for years, can barely write an article not denigrating Trump, also opined on drug safety, calling Trump’s followers “hydroxychloroquine kooks” adding his smarmy epithet for Trump’s medical experts (ostensibly including Yours Truly) as a “coterie or bootlicking minions” [SIC]. Montini trusted regurgitated propaganda from other lay reporters regarding the Lancet and the New England Journal of Medicine as nothing short of biblical wisdom, beyond any deliberation, discussion, or critical analysis.
- Former independent presidential candidate Robert F Kennedy, Jr (RFK) was also called out for his promotion of HCQ. RFK was chastised as recently as a few days ago, on August 23, 2024 by “fact-checkers” at the putatively venerable University of Pennsylvania in a piece titled: “RFK Jr.’s COVID-19 Deceptions” which stated “As we’ve written, clinical trials of both ivermectin and hydroxychloroquine have shown no evidence of effectiveness against COVID-19”…despite the analysis of hundreds of HCQ studies involving hundreds of thousands of patients detailing the exact opposite and with high statistical probability. How peculiar that not even one scientist or physician from University of Pennsylvania’s Perelman School of Medicine (which incidentally has dedicated epidemiology and biostatistics faculty) were seemingly consulted, referenced, or quoted anywhere within its “fact-check” declarations, especially since the authors do not appear to have formal statistical/ epidemiological/medical/pharmacology/pharmacy training themselves.
Timeline of the Distortion Hydroxychloroquine Safety, Almost Immediately Following Trump’s Proposal:
In case anyone has forgotten about the actual, carefully worded declarations that President Trump made, delicately suggesting that data had shown that HCQ could be useful for Covid-19, here are his actual statements made during his press conference:
Trump directly stated during a press conference on March 20, 2020 that he proposed to use HCQ for early treatment “at the beginning” of Covid-19 infections at 0:22 in the video above. Trump was right to suggest that because today, there is a great deal of evidence that early (or even prophylactic) treatment with HCQ is very effective for Covid-19.
During the same March 2020 press conference and standing alongside Trump, Fauci very accurately stated that “[HCQ] toxicities are rare, and in many respects, reversible” at 1:50.
Following Trump’s proposal and subsequent stockpile of product, HCQ experienced a stunning, seemingly coordinated, fall from favor.
First, Fauci changed his mind regarding his March 2020 statement following the publication in the New England of Medicine on May 1, 2020 (later retracted), the FDA’s problematic declarations in its review on May 19, 2020 (later retracted), and the Lancet’s publication on May 22nd, 2020 (later retracted).
Despite historical evidence of HCQ being both safe and effective, physicians, politicians, and organizations, taking their lead from incorrect narratives from Fauci, the press, and medical publications, rushed to parrot outrageously incorrect anti-HCQ on top of highly emotional anti-Trump narratives.
The following are just a few of the dozens upon dozens:
- Four months after stating the exact opposite during the above press conference video, on July 29, 2020, Dr. Anthony Fauci told CNBC that there was “no evidence it was effective.” Fauci also suddenly shifted, adding that HCQ for Covid-19 “didn’t make scientific sense.” Seemingly not critically evaluating or looking beyond (falsified and now retracted) New England Journal and Lancet conclusions.
- Josh Cohen, a Forbes.com PhD senior healthcare columnist (with a background in economics) later headlined an absurdly biased op-ed based on a study out of France stating that Trump’s HCQ proposal could be “Linked To 17,000 Deaths” …except Cohen left out the “could be” part. Forbes’s Tufts- Harvard- and University of Pennsylvania- trained “healthcare analyst” (who also opines on climate change and the Inflation Reduction Act) fully neglected to mention that such a number was fully theorized, conjectural, and that estimation was an extrapolation of a late-stage “compassionate use” scenario where HCQ could have, hypothetically caused an 11% increase in mortality. He also chastised the “unproven, experimental nature of hydroxychloroquine” even though non-experimental and proven positive clinical findings existed prior to his piece being published. Of course, one would never know that by Forbes’ appallingly misleading article title which instead of being an “analysis” seemingly closely paralleling the writings of non-”healthcare analysts” at The Hill and Politico. The original publication upon which Cohen’s (and The Hill’s and Politico’s) article was based, was (in what has become a familiar pattern) retracted at the request of the publishing journal’s editor-in-chief following an investigation of journal subscribers which uncovered major deficiencies in the study’s dataset (and other failings). Despite that, the above (in addition to other) news articles remain live and online, accumulating hits from a public that believes them to be factual and current, still used as a talking point against Trump. Will redactions of these badly outdated op-eds ever take place?
- Physician and outspoken medical pundit Dr. Vinay Prasad, a University of California at San Francisco oncologist stated via a MedpageToday.com op-ed: “And, yes, let’s put it to bed: hydroxychloroquine doesn’t work for COVID,” while lamenting within that same MedPageToday article that anyone was even permitted a voice to be given to an opposing side in the Covid-19 debate. Like others, Prasad had slammed the door shut following preliminary findings. Prasad seemed to perform rote parroting of the opinions of others in place of a comprehensive or original review of the data as one would expect from an outspoken physician. In the same article, he also compared giving a voice for alternate Covid-19 treatments like HCQ to giving a voice to “flat-earthers.” Dr. Prasad also stated via a Washington Post op-ed that “Trump’s medical judgment is wrong. The example he’s setting is worse.” He also stated via Twitter/X that “The prior administration loved hydroxychloroquine and ivermectin and other foolish unproven drugs.”
- Yale University’s Department of Epidemiology’s Dean, Dr. Stan Vermund quoted a line, admonishing against the use of HCQ for Covid-19 on July 29, 2020 stating “[HCQ] showed no benefit for decreasing the likelihood of death or speeding recovery.” His widely-cited letter remains online, prominently showing up in internet search results to this very day. Dr. Vermund was chastising HCQ research published by Dr. Harvey Risch, a distinguished epidemiologist and Yale colleague, while failing to directly link Dr. Risch’s publication and also not specifically listing any fault with Dr. Risch’s research methodology. At the time, well-conducted studies contradicted Dr. Vermund’s quote and sentiment against HCQ, with dozens upon dozens more studies since released, but no updated or revised letter from Dr. Vermund addressing those favorable data.
- The WHO stated that HCQ “has no meaningful effect on deaths or hospitalizations” and “made a strong recommendation against the use of hydroxychloroquine” and that they “…do not consider this drug worthwhile.”
- The WHO later stated in March of 2021 that “more than 80 trials planning to enroll at least 100,000 participants to further research hydroxychloroquine are unlikely to uncover any benefits and should be canceled.” It appears that the WHO’s mind was made up, and that even potential new data examining different doses/endpoints/HCQ timing/study design, were seemingly pre-determined to be immaterial and confirmatory data irrelevant. (Of note, after the New England Journal of Medicine and Lancet studies were retracted, the previously halted WHO studies that were able to resume, did.)
While there was an exuberant and highly publicized effort to denigrate Trump’s HCQ initiative, there was little to no effort to critically evaluate what was being said, correct the records, or communicate the objectively wrong quotes and narratives regarding HCQ following article retractions or as new data emerged contradicting old data.
After the Government/Medical Journals/Media Failed, So Did Hospitals and Academia:
Covid-19 was a fundamental test of medical science, but instead of taking the time to slow down decisions and to stop, focus, and rely on objective, scientific, and clinical fundamentals, America’s trusted federal officials, physicians, and scientists panicked and instead enthusiastically leapt to expensive, novel “warp-speed” solutions on the advice of commercial, for-profit companies. Shockingly few pushed back, and those few that did were silenced. That behavior loosely aligns with 2019 data showing that 91 percent of medical prescribers reflexively believe that FDA-approved products are completely safe and always benefit patients.
As has been made clear with novel Big Pharma Covid-19 approvals, people can manipulate scientific data to promote a narrative, but continued accumulation of factual research, combined with ancillary findings, and the manipulation eventually becomes increasingly difficult, if not impossible. Scientific truth eventually shines through. Detailed evidence in the form of narrative-contrary epidemiological findings regarding both the safety and efficacy of Covid-19 products eventually became apparent.
The true academic medicine scientists didn’t blindly take marching orders from journalists, the WHO, federal agencies, Anthony Fauci, state governors, or anyone else. We methodologically and objectively examined the data and let the accumulated real-world clinical findings speak for themselves. It’s the objective attitude that every scientist should have followed, instead of rushing to conclusions – pandemic or not – madly grasping at the first, shiny, new Big Pharma thing, and then quickly permitting it upon their patients and fellow countrymen through mandates, employer requirements, or financial motivations.
Physicians and Pharmacists Persecuted for Prescribing and Dispensing HCQ:
Clinicians like myself who advocated for any alternate treatments such as ivermectin or HCQ were mocked online by non-medically- and non-scientifically trained “trusted journalists” and “fact-checkers” as being part of a “right-wing conspiracy.” Anyone who didn’t demure to the Covid-19 mRNA or other Big Pharma Covid-19 treatments and narratives were banned, fired, and blasted as “anti-science” around the world and into the reaches of the stratosphere via the internet. And if that wasn’t outrageous enough, it didn’t end there.
After physicians and pharmacists lost their jobs, their reputations, practices, insurance, finances, licensure, and careers were destroyed. That’s because in many cases, even after losing their jobs, state medical and/or pharmacy boards with broad and vague authority plus seemingly limitless taxpayer-funded budgets initiated legal proceedings against their licensure, cherry-picking the persecution of their “off-label” Covid-19 treatments (including ivermectin and HCQ) when other “off-label” treatments for non-Covid-19 diagnoses were a near-ubiquitous component of almost every medical and pharmacy practice. On top of that, America’s press and “fact-checkers” singled out and sought to embarrass providers through online articles.
Starting in May 2024, Americans learned through a Republican House Judiciary report and Elon Musk’s purchase of Twitter that Facebook, YouTube and Amazon, that a great deal of Covid-19 narrative, punishment, and censorship was directly coordinated by the Biden White House via direct legal threats.
Appallingly, it was America’s White House that forced private companies to censor objective facts on now-proven to be effective repurposed treatments (including HCQ) while simultaneously advocating for and/or mandating the use of novel, expensive Big Pharma treatments, and the press were just tools to advance and reinforce the White House’s censorship.
TIMING MATTERS When It Comes to Successful Hydroxychloroquine Treatment for Covid-19:
Negative data surrounding HCQ seemed to be restricted to the US and other Western countries. HCQ was shown to be useful for preventing initial Covid-19 infection when employed as an early treatment protocol, which was why HCQ (or chloroquine) was adopted for Covid-19 treatment during the initial pandemic period, at least in part, by 42 countries (58 countries when including non-government medical organizations).
Most negative studies cited by the American government, academia, and Big Pharma officials ignored a very basic but critically important pharmacology fundamental: Any antimicrobial pharmacology (including: antibiotic, antifungal, antiviral) is substantially less efficacious when it’s implemented during the late-stages of infections, at which point the rapidly replicating infection would overwhelm an individual. Early/immediate treatment is the clinical standard for the treatment of all viral infections, regardless if the virus is: influenza, cold sores, HIV, or Covid-19. Timing is especially important to consider when treating the elderly/infirm.
Despite that, early treatment was ignored in an abundance of “top-tier” peer-reviewed medical journals, including (once again) America’s New England Journal of Medicine. Two prominent, highly-cited, examples are shown below:
SOLIDARITY New England Journal of Medicine (Bibliography #377)
In June of 2020, the New England Journal of Medicine published a poorly designed SOLIDARITY trial denigrating HCQ. HCQ findings were negative because SOLIDARITY study coordinators employed HCQ late treatment methodology to treat Covid-19 patients, despite early treatment being the clinical standard of care.
SOLIDARITY was an open-label RCT (no placebo control arm) trial which showed: 19% higher mortality (p=0.23). SOLIDARITY used 954 very late stage, critical (64% of patients were already on oxygen/ventilation) patients to administer HCQ. Data showed a spike in HCQ mortality at days 5-7, corresponding to about ~90% of the total excess mortality. Almost all excess mortality in this study originates from those very late-stage ventilated patients administered HCQ. HCQ dosage was also exceedingly high and study coordinators did not appear to adjust doses based on patient weight, meaning that potentially toxic dose concentrations from higher doses may have occurred in patients with lower weights.
The WHO authors refer to a lack of excess mortality in the first few days to suggest a lack of toxicity, but seem to fail to consider the very long (approximately 40-day-long) half-life of HCQ. The pharmacology/pharmacokinetics of chloroquine metabolism are complex, with the half-life increasing with increasing dosage. Additionally, an unspecified percentage of patients were administered the relatively more toxic chloroquine as an alternative to HCQ.
RECOVERY New England Journal of Medicine (Bibliography #383)
RECOVERY (Randomized Evaluation of COVID-19 ThERapY) trial published December of 2020 found no significant benefit for very late stage, (9 days after symptom onset) in already very sick patients since viral replication had already overwhelmed patients. As in the SOLIDARITY trial, late treatment for Covid-19 or other viral infections is not the standard of care.
Negative results could have also been due to toxicity from the unusually high dosage used (9.2g total over 10 days) which had been shown in the past to be associated with an increase in risk. Authors did not report results based on weight, BMI, or comorbid underlying conditions such as diabetes and HCQ should be dosed based on weight. Like SOLIDARITY, authors did not adjust HCQ dosage based on patient weight, meaning that toxicity may have been higher in patients of lower weight. Data showed a spike in HCQ mortality on days 5-8, corresponding to ~85% of the total excess seen at day 28 (a similar spike is seen in the SOLIDARITY trial).
Authors note: “we did not observe excess mortality in the first 2 days of treatment…when early effects of dose-dependent toxicity might be expected” but they failed to consider the high dose used on top of the approximately 1,000 to 1,200 hour half-life of HCQ. Administering a drug on a daily basis with a long half-life means that much higher levels of HCQ will be reached later on, as it accumulates. Additionally, patients in this trial were late treatment and extremely sick (median 9 days post symptoms, 60% requiring oxygen and an additional 17% requiring ventilation/ extracorporeal membrane oxygenation (mechanical oxygenation of the blood, extremely high-risk medical intervention with 50% mortality). An unusually high mortality rate was seen in both arms: 1,561 HCQ patients, 3,155 standard-of-care. An additional breakdown of RECOVERY detailed a significant, lengthy list of RECOVERY methodological inconsistencies. (article was internet “auto-translated” from French)
Hydroxychloroquine Reliably Beneficial in Early Treatment Studies:
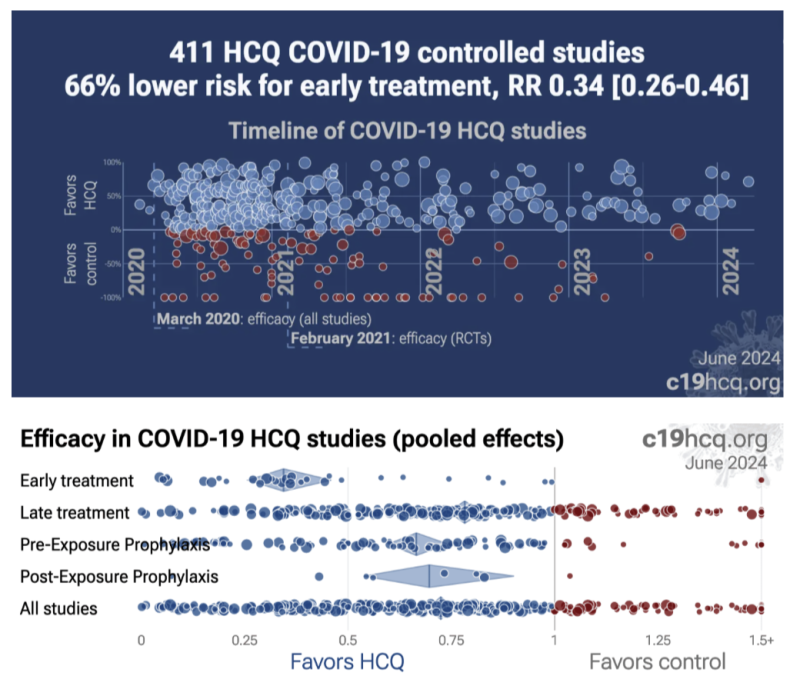
Despite early treatment being the standard of treatment, it was systematically ignored by investigators, “top-tier” journal peer reviews, and the press. According to a breakdown study analysis of 39 early treatment studies at c19early.com, they showed a dramatic 66% [range: 54-74%] lower risk. Seventeen of those 39 studies show a 76% [61-85%] lower mortality and 16 studies show a 41% [28-51%] lower incidence of hospitalizations.
Late-treatment implementation was also successful, but less so, with 22% [18-26%] lower risk from 264 studies. Very late treatment tended to be not useful and even harmful, especially at excessive dosages – as would be expected with just about any drugs given at excess doses to late-stage patients of any kind.
To visually illustrate this effect, here are two comparisons detailing early treatment studies (Row 1) versus all HCQ study findings (Row 2). While HCQ is generally associated with favorable outcomes, early treatment studies have the most favorable outcomes, (as is typically the case for other antimicrobial pharmacotherapies). Negative outcomes unquestionably occurred but they were typically the result of treatment delay, diagnosis delay, incorrect dosing/duration, and/or otherwise attempting to treat Covid-19 after viral replication had uncontrolled replication for days.
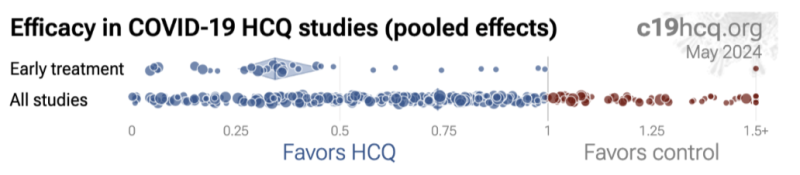
Late Treatment and Incorrect Doses Incorrectly Translated as Hydroxychloroquine Being Harmful and/or “Not Effective:”
Widely disseminated press reports of negative conclusions associated with HCQ reflected the inappropriate timing of HCQ in the form of “late treatment” (or sometimes even very late treatment studies) and/or studies that tended to disregard/not specify an HCQ treatment delay following diagnosis confirmation. The lay press, not knowledgeable in medical pharmacology or treatment standard fundamentals, ignored HCQ timing, dosing, duration, and other important caveats when parroting negative tropes against Trump and his proposals for HCQ. Their narratives originated from what appeared to be coordinated messaging and “top-tier” journals and their expert “peer-reviewers” who in turn seemed to also overlook key issues with HCQ dose, duration, and/or delayed administration.
Well over half of all HCQ clinical studies in the bibliography below ignored the clinical standard of early administration and meeting the definition of less-effective, late treatment – and yet when collectively compiled as part of the meta-analysis, HCQ still demonstrated a somewhat overall (22% [18 26%] lower risk) positive effect.
Specific treatment delays are specified in the full bibliography and their individual accompanying summaries.
General Negative Study Bias in Hydroxychloroquine Publications?
One of the biases from the press and federal officials may have been due to more heavily weighing of seemingly biased studies published in the United States. Curiously, at least one review of data showed that HCQ studies from North America were found to be 2.4 times more likely to report negative results than all studies from the rest of the world, combined.
It showed that the same studies which had negative results associated with HCQ (in late 2020 as Trump was in office promoting HCQ) were correlated with medical/scientific authors having a history of giving donations to the Democratic Party.
That sort of potential study bias is something which would normally warrant vigilant scientific investigation, especially because taxpayer money likely directly or indirectly funded at least some component of almost all American clinical research. Despite that, no investigation has even been proposed, much less carried out.
What about Cochrane’s Review of Hydroxychloroquine?
While Cochrane reviews are and often relied upon reference for the cumulative analysis of many studies, Cochrane HCQ analysis only reviewed 14 studies, (and only analyzed 12 of them). Cochrane’s last review of the data was back in September 2020, ignoring >90% of existing HCQ data. When will Cochrane update its review to include the HUNDREDS of subsequent clinical studies? Who knows, but that is where c19 early analysis comes into play which duplicated Cochrane’s methodology (DerSimonian and Laird random effects model), but expanded it to include all available clinical studies, providing a definitive, up-to-date answer. The full list of the 400+ HCQ clinical studies examined is included in the bibliography.
Along with Cochrane, the press seems to have deliberately ignored a large volume of clinical data both then and now; instead, employing select results to match an anti-HCQ, anti-Trump narrative. HCQ (along with several other repurposed treatments) should have been objectively considered and/or implemented as initial treatments and/or non-placebo alternates, and/or comparators to mRNA technology or against other expensive, novel Covid-19 treatments, including Paxlovid and Remdesivir.
Based on these data, it appears likely that HCQ would have been superior from both a safety and efficacy standpoint – and with Trump’s “donated” supply – free.
Free, Donated Hydroxychloroquine vs Expensive, Novel Remdesivir Study Biases:
While ironically ignoring positive findings of HCQ and implementing early treatment methodology standards ignored with HCQ, remdesivir was approved and endorsed by the FDA for the treatment of Covid-19 based on an April 2020 study that yielded no positive results.
Despite that, the FDA approved remdesivir anyway, and without even consulting their own appointed advisory committee.
A similar expedited approval took place in the EU, just before the disappointing WHO trial data was released, and apparently while the disappointing trial results were known to the manufacturer. Gilead’s aggressive marketing campaign proceeded despite questionable efficacy and the lack of transparency in both the FDA/EU approvals.
The SOLIDARITY trial, which also contained an arm which examined remdesivir, showed that remdesivir did not reduce mortality or decrease the time that it took for patients to recover from Covid-19.
The preponderance of the cumulative, up-to-date findings illustrates that there are no statistically significant or clinically meaningful improvements with remdesivir use.
In the few positive studies, the small non-significant mortality improvement disappears with longer follow-up duration. Despite all of that, hospitals were financially incentivized to entice patients to use remdesivir by granting a mysterious 20% “boost” bonus payment from Medicare on the entire hospital bill for those patients who agreed to receiving remdesivir and larger bonus payment to the hospital if a Covid-19 patient is mechanically ventilated. In the end, that worked out to each hospital receiving “at least” a $100,000 “bonus” per patient, paid for by American tax dollars.
Unlike fully transparent and available HCQ studies for anyone to examine, Remdesivir study data and official messaging has been described by scientists as confusing, unfair, incomplete, and untransparent. Study findings detailed 8.6% more deaths in the Remdesivir group than in the placebo group. The results of that study, showed on day 28, 7.2% (22 out of 158) in the Remdesivir arm died, while 7.8% (10 out of 78) in the placebo arm died.
Remdesivir studies also had the metric of “death” removed from its primary endpoint in what is now a familiar pattern of the FDA failing to warn Americans about adverse events while permitting Big Pharma to skew drug safety data collection methodology by simply not requiring collecting it. The difference in death rate, one of the original primary measures, was not statistically significant, showing only a marginal reduction from 11 percent in patients given a placebo to 8 percent in patients given remdesivir.
The available clinical data did not support remdesivir approval, let alone a strong White House endorsement, let alone a federally sanctioned payment incentive for hospitals, from taxpayers. The confusion regarding both the approval of and the financial incentive borne by taxpayers perplexed enough people to the point that it was even criticized in non-medical publications such as science.org.
Bottom line: Remdisivir was (probably) not safe and not effective, even though it was tested against “early treatment” patient parameters, in contrast to HCQ studies, and still showed negative findings. It was then expedited for approval by FDA officials and medical reviewers. Unlike Trump’s donated HCQ which had transparently positive evidence for benefit in early treatment, Remdisivir had extremely limited clinical history (versus HCQ’s 55 years of clinical history), was not efficacious, and less safe, and because of some unclear deal, had a significant financial incentive encouraging its administration, in turn making hospital bills substantially more expensive.
Four Years Later: Persistent, Incorrect, 2024 Claims of “Hydroxychloroquine Doesn’t Work for Covid-19”
As of this very day, there are some major medical centers, medical schools, and other organizations that still have operating webpages, very conspicuously appearing in the first page of internet search results, actively continuing to advocate for remdesivir while simultaneously regurgitating incorrect narratives about how HCQ should not be used for Covid-19.
All of the below results showed up very prominently (on the first page of results) following a routine internet search for the facility name and the terms “hydroxychloroquine covid” during July 2024.
Despite an abundance of objective, real-world data fundamentally stating otherwise for years now, for many people the Big Pharma/White House narrative scales will never fall from their eyes. Indeed, there are none so blind as those who will not…examine the clinical evidence and compare assessment methodologies.
Specifically: here is a list of major medical centers that are still parroting outdated, incorrect tropes regarding HCQ. Although all of the website statements below are incorrect, some are “more incorrect” than others as Orwell might have said. Here are just a selection:
- Mayo Clinic: “[HCQ] is not recommended as a treatment for coronavirus disease… Also, hydroxychloroquine doesn’t prevent infection with the virus that causes COVID-19.”
- Cochrane Policy Institute: “[HCQ] does not reduce deaths from COVID-19…”
- Drugs.com: “[HCQ] does not provide a medical benefit for hospitalized patients with COVID.”
- Honor Health Hospital Network: “Question: Should I have [HCQ] on hand in case I feel sick?… Answer: No.”
- Cleveland clinic: “Overall, [HCQ]…has never been shown to be helpful in fighting COVID-19.”
- Duke University Medical Center: “Hydroxychloroquine is the most disappointing, disavowed drug that researchers keep studying for COVID-19.”
- Baylor University Medical Center: “Randomized controlled trials have repeatedly shown that [HCQ] is not effective to treat or prevent COVID-19.”
- Baylor’s Dr. Peter Hotez: “Ivermectin does nothing to help people with Covid, same with [HCQ].”
- Kaiser Permanente Health: “[HCQ] is not recommended for coronavirus infection…unless you are enrolled in a study.”
- Houston Methodist Hospital who suspended HCQ advocate Dr. Mary Bowden: “[her] opinions are harmful to the community, do not reflect reliable medical evidence or the values of Houston Methodist.” Hospital spokesman went on to say Dr. Bowden was ‘spreading dangerous misinformation not based in science.’”
- U.S. Food and Drug Administration: “[HCQ has] not been shown to be safe and effective for treating or preventing COVID-19.”
The FDA link immediately above goes on to reiterate the established cardiac adverse events and drug-drug interactions associated with HCQ use, as per its safety review memorandum discussed earlier.
Abundance of Scientific Data Shows Safety and Effectiveness of HCQ for Covid-19:
Much like two consecutive Cochrane-reviewed scientific studies showing that all widely-mandated masks, including surgical masks, and N-/KN-95 masks are almost certainly ineffective for inhibiting Covid-19 transmission, study data trickled out over time about the benefits of HCQ soon after Covid-19 started spreading. Those findings eventually accumulated into the avalanche of clinical evidence before us today, illustrating that HCQ is objectively effective for Covid-19 prevention and treatment. It is no exaggeration to say that HCQ would have helped many millions.
To clarify the evidence, while I have a scattered mess of HCQ studies on my computer, office and bedroom, dog-eared and food-stained, there is an elegantly-presented meta-analysis which employs the same analytical methodology that Cochrane reviews use. It details how the compilation of: over 400 studies, conducted by over 8,000 scientists, involving over 525,000 patients across 58 countries, showed that the appropriate clinical utility of HCQ for Covid-19 resulted in a statistically significant lower risk for 1) mortality, 2) hospitalization, 3) recovery, 4) cases, and 5) viral clearance.
Of note, this was not just a cherry-picking slash Texas sharpshooter fallacy of select data findings; it represents a compilation of all available clinical data.
Reviewing and Evaluating All Available Clinical Studies:
In composing an argument, one needs to consider all available legitimate data – not just select summary findings parroted by news reporters or exclusively relying on findings from selected “top-tier” medical journals. It is no secret that “top-tier” journals accept significant sponsorship from Big Pharma to cover its expenses, which now include ancillary, non-sequitur agendas such as an experimental “artificial intelligence division.” As has become clear in the recent decade, and most apparent under Covid-19, studies published in “top-tier” journals including the New England Journal of Medicine, The Journal of the American Medical Association and the Lancet are not Holy Scripture above critique, and can actually be dead wrong.
That’s why it’s important to get corroboration from alternate sources. There are very legitimate clinical data being published, including data from other countries and/or data published in smaller journals (without Big Pharma sponsorship) worthy of clinical and epidemiological consideration. In fact, academics who spend their lives in medical research will tell you that non-big-name, smaller studies, observational, and/or real-world study data when examined in combination are not only very worthy of consideration – but may be more reflective of a drug’s efficacy and safety. In other words, the totality of evidence from multiple, well-designed, and smaller, real-world, case reports, case series, and/or observational trials can actually be a stronger indicator of a clinical/statistical effect than that of just one or a few biased large trials.
To-date there are over 400 clinical studies examining HCQ use in Covid-19 with both negative and positive findings. A complete bibliography of all studies, and study summaries examined are provided in the form of an annotated bibliography at the end of this article.
The list and review of the data and the bibliography excluded studies known to be the product of fraudulent research, including those by Elshafie, Dabbous#1, Dabbous#2, Abd-El-Salam, and the aforementioned Desai Lancet and New England Journal of Medicine publications.
The Good and Bad of Large Randomized Controlled Trials (RCTs):
Randomized Controlled Trials (RCTs) are conceptually preferred if they are properly designed and conducted. However, the Covid-19 era exposed critical biases in such trials, including but not limited to: treatment delays (any antiviral treatment for any viral infection, including Covid-19 must begin promptly), protocols that were designed to fail, mid-study changes to the study protocol, biased analysis and presentation, lack of transparency in data, and suspiciously timed publication releases.
As has been shown here, biases on top of important study design and standard-of-care treatment shortcomings can lead to wildly incorrect clinical study conclusions. Every HCQ clinical study needs to be evaluated on individual merit for potential biases and/or confoundings, whether randomized, real-world, observational, large, or small trials.
Large RCTs allegedly producing the Big Pharma-generated-idiom of “Evidence Based Medicine” published in “top-tier” journals often appear very compelling – especially because they are what the lay press tends to focus on – but in the recent past, it has become clear that responsible clinical scientists must very carefully examine methodology used beyond the high-level summary overviews, and to also look at additional non-RCT sources of data for confirmation of findings.
Another problem with large RCTs is that unlike real-word and observational studies, not just anyone can conduct large RCTs. Barriers include them often being significantly more expensive, time-consuming, and requiring a dedicated, highly skilled support staff. That prevents less-well-funded clinicians who have smaller practices/facilities or clinicians that have employment requirements which have a focus on direct care responsibilities as opposed to clinical research.
While federal grants are available for RCT endeavors, those grants are highly competitive and tend to be limited to particular diseases or topics which in turn end up being awarded to particular facilities with the aforementioned support staff, infrastructure, et cetera. Those major centers and/or their employees tend to be connected in one way or another to Big Pharma funding.
When Covid-19 emerged, billions of taxpayer dollars were given to Big Pharma. That kind of trust seems to have beguiled unethical physicians and scientists to create an incentive to show a lack of effectiveness or safety for inexpensive generic products, while in turn show efficacy for a novel, expensive, patented commercial product as a way for obtaining even more taxpayer money. Big Pharma scientists can be motivated to show benefit to their product versus existing, less expensive, or generically available technology. This scenario not only applies to Covid-19 treatments such as HCQ or ivermectin, but to a fair amount of all investigational medicine research.
These sorts of biases can lead to discordant findings in RCT versus real-world clinical findings. As is the case with discordant HCQ findings, and/or other studies related to Covid-19 approvals, it’s important to investigate the reasons as to why. Unfortunately, it appears that there is little to no FDA/CDC/NIH or White House appetite for unearthing the truth. The evidence shown here is a preliminary concept into what an investigation ought to examine.
You Can Run (With a False Narrative) but You Can’t Hide (From the Data): Analyzing All Available Hydroxychloroquine Clinical Data:
A meta-analysis combines studies to perform a broad analysis. This type of methodology is accurate, valid, and widely respected by epidemiologists, statisticians, and other medical/scientific disciplines. In fact, the present analysis of HCQ in the bibliography here employs the same analysis methodology that Cochrane routinely uses to provide a complete picture of an effect across studies.
These statistical findings from clinical studies are in addition to the highly plausible molecular biology and pharmacological mechanisms of how HCQ is effective for preventing the entry of many viruses into cells. For purposes of keeping the length of this review manageable, the pharmacologic mechanism of action of HCQ will not be discussed here.
Comprehensive Meta-Analyses Evaluating all Findings (Both Good and Bad) Show Hydroxychloroquine’s Benefit:
Meta-analyses which combine RCT and observational/real-world-use studies across many facilities make the strongest case. The dependence on any individual trial is subject to potential confounding, shortcomings, errors, bias, incompetence, and even fraud.
A diagram adapted from a Nature publication below illustrates a scenario in which four smaller studies that may not have delivered statistical significance individually (ie, have a p>0.05), but could show strong evidence with a statistical significance when analyzed in combination via a meta-analysis:
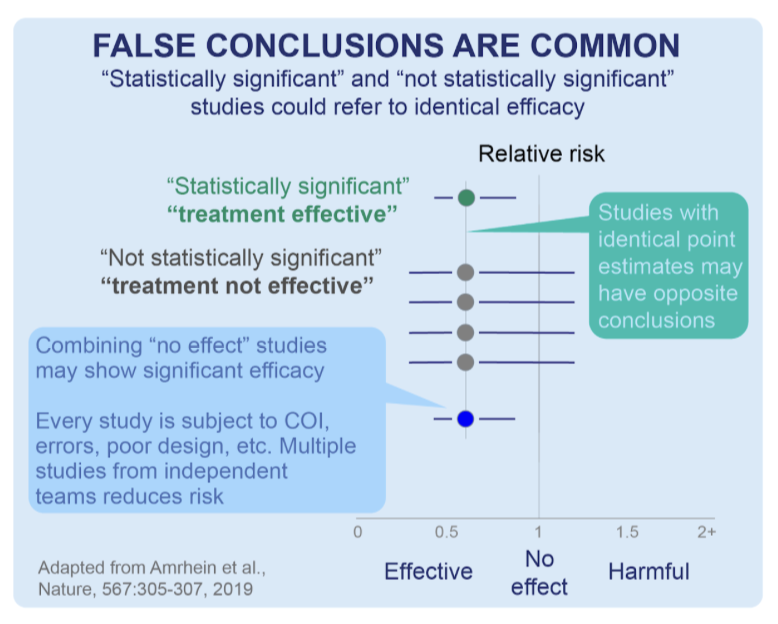
To date, the DerSimonian and by the random effects meta-analysis model conducted by the c19early analysts shows a clinically beneficial effect of HCQ for Covid-19 treatment with a certainty of p<0.00000000001 (that is, one in one sextillion) over all >400 HCQ studies.
RCTs for specific outcomes like mortality, hospitalization, and recoveries each show a very strong benefit with a p<0.0001.
The beneficial effect of HCQ includes delayed treatment and other negative result studies, despite late treatment being significantly less effective. Treatment delay and/or late (and sometimes very late) treatment was implemented in over half (n=264) of the HCQ studies in the bibliography below. Of note, a high number of late/very late/delayed treatment studies compiled into the meta-analysis still ended up showing some beneficial effect of HCQ administration, illustrating its strong efficacy. Potential confounding factors may include viral replication, viral loading dose, viral variant/mutation, on top of numerous demographic, immunologic, and other factors. Avoiding treatment delay is a fundamental concept taught early on in both pharmacy and medical schools.
Financial Ramifications of Condemning Trump’s Hydroxychloroquine Proposals in Favor of Big Pharma Alternatives:
While Trump’s proposal to use HCQ was negatively bombarded, novel, expensive Big Pharma treatments with very limited data were developed, (and tested against placebo instead of treatments including HCQ or ivermectin) and rapidly reviewed, authorized by America’s FDA and purchased with taxpayer debt by the Biden White House. Despite limited findings, Paxlovid ($1,400 per treatment course), Remdisivir ($3,120 per course), and Molnupiravir ($700 per course) were White House-endorsed despite Trump having already secured HCQ for free. By the end of 2021 alone, the White House had already spent over $10.6 billion just on Paxlovid alone and subsequently purchased more. All of the White House’s Covid-19 treatments were lacking long-term efficacy/safety findings as compared to HCQ.
For perspective: the greater than $10.6 billion the government spent on Paxlovid just through 2021 alone could have instead purchased about 353,000 $30,000 Toyota Camry SEs (the most popular model) for destitute Americans who lost their cars due to the economic downturn.
Even worse: according to the most recent findings, (and like remdesivir) Paxlovid doesn’t work, even if you double the length of dosing according to the most recent and cumulative findings published in the July 2024 issue of the New England Journal of Medicine.
It reconfirms an earlier published case report just weeks after Paxlovid was approved and given to Americans showing that people who take Paxlovid don’t get better sooner, compared to those taking placebo. The medical community has known, and written about Paxlovid rebound which occurred from the very beginning.
Of note, rebound from HCQ is significantly less likely to occur because of its very long, aforementioned half-life.
Today, even the White House-fawning press is openly mocking the use of Paxlovid for Joe Biden’s July 2024 infection with Covid-19 in both the title and photo caption from Business Insider below:

Between $16 and $22 Trillion Wasted:
Trump’s donated HCQ for pre-exposure prophylaxis, early exposure, or early treatment (in eligible individuals), would have worked better than Paxlovid and could have also been used to prevent the numerous strains of Covid-19 from the beginning.
And the tens of billions of dollars wasted on Paxlovid and other Big Pharma boondoggles were chicken scratch relative to the entire cost of the pandemic.
It’s been estimated that the Covid-19 pandemic has cost Americans at least $16 trillion according to Harvard economic researchers, $18 trillion according to Heritage Foundation scholars, with other estimates being even higher from the Institute for Progress. It’s hard to imagine how much even $1trillion is, but here is one example relative to seconds or days. Relative to automobiles, using Harvard’s lowest estimate of $16 trillion, that amount of money could have instead bought a new $30,000 Toyota Camry SE for every single American citizen (man, woman and child of any age) in America with over $5 trillion left over. Instead, Americans are not only not getting a new Toyota Camry SE, but are instead losing the cars they do have, losing their homes, and are otherwise being crushed by high inflation on just about everything they need, including food, gasoline, baby formula, and electricity.
It is no exaggeration to state that Trump’s HCQ proposal could have prevented much of the negative financial, social, and psychiatric ramifications of Covid-19 – not to mention morbidity and mortality. According to the meta-analysis of the studies in the bibliography below, HCQ would have been effective and could have potentially avoided the vast majority of its $16 trillion expenditure.
The bottom line is: President Trump was correct to secure a donation of, and advocate for the use HCQ for eligible individuals. The most recent cumulative positive findings associated with HCQ are undeniable evidence that Americans would have been better off had HCQ had been implemented and used in eligible populations.
Hydroxychloroquine Summary Data Graphs:
To fully address transparency, I am including a full list of HCQ studies completed to date which comprise the meta-analysis showing HCQ’s efficacy and safety. Each of the 400-plus references include a brief summary and a link to a longer analysis at c19early.
The bibliography includes all clinical data including both positive and negative findings that implemented wrong dosing, too-short-duration, and studies which employed late treatments. It also included studies not reaching statistical significance (p>0.05). Hyperlinks to the original studies data are also provided.
In some cases, journals held clinical studies from publication for an extended period and then rejected papers without review (some journals still refuse to defy Big Pharma sponsors and/or may have been threatened to censor their data by order of the White House). For others, authors could have lost authorization from their employer to publish, or they may no longer want to pursue journal publication for fear of negative impacts on their careers or personal or employer funding. It’s also a distinct possibility that some authors simply deprioritized advancing publishing and moved on to other research or clinical duties required of them when Covid-19 morbidity and mortality collapsed following emergence of the Omicron variant (in late 2021) along with the downstream variants thereof.
Along with the bibliography, I am also including several HCQ scatter plots illustrating the number of negative versus positive findings from c19early on overall benefit, and breakdown of relative benefits from prophylaxis, early and late treatments.
Summary:
“Tip-tier” medical journals, mainstream press, hospitals, administrators, insurance companies, Big Pharma, state/municipal government, on top of every federal alphabet agency and others all converged to a singularity of promoting a grand illusion of manufactured consent that demonized HCQ while favoring novel, minimally tested, minimally effective, expensive commercial Covid-19 treatments. The linked meta-analysis now proves HCQ’s effectiveness. It’s no exaggeration to state that the manipulation of data that occurred with HCQ (and other repurposed drug treatments) was the biggest scandal in the history of American medicine, and one of the biggest medical crimes against humanity.
The mission of science and scientists is to cultivate critical thinking coupled with a willingness of its disciples to adjust their thinking and admit being wrong about existing ideas or theories. In other words, recognizing that no science is ever entirely “settled” and therefore should not be silenced.
- Confirmation bias in referencing select HCQ RCTs was not science.
- The FDA’s safety memorandum on HCQ involved inappropriate, cherry-picked data and was not science.
- Ignoring the standard of care and mostly considering late and very late and inappropriate doses/durations of HCQ treatments as a means to denigrate Trump’s HCQ proposal was not science.
- Silencing medical and scientific experts critical of the White House, FDA, and Big Pharma was not science.
- The lay press’s incompetent evaluation and parroting of highly flawed HCQ data was not science.
- Articles from The Hill, Forbes’s, and Politico which rushed to amplify critique on HCQ and Trump – but following study retraction, keep their articles online and continually accessed by the public, was not science.
- Medical journals not demanding lay-press corrections on updated/retracted findings based on its internal “peer-review” failures to verify non-sequitur HCQ findings was not science.
- Hospital narratives regarding HCQ published on public-facing websites was not science.
- The failure of “peer reviews” at “top-tier” medical journals to consider established clinical treatment standards of early treatment was not science.
- Allowing non-medically trained “fact-checkers” to comment on medically and technically complex aspects of pharmacology and medicine was not science.
- Punishing community pharmacists and physicians for appropriately choosing to dispense and prescribe HCQ for Covid-19 was not science.
- Demanding a single “consensus” on how pharmacists and physicians were permitted to treat Covid-19 was not science.
- A federal official (or any one person) referring to himself uniquely as “the science” was not science.
Thousands of well-educated scientists and clinicians in America’s federal government, universities, and hospital settings ignored the historical, careful scientific evaluation process established over millennia by their scientific predecessors. No falsehood regarding HCQ for Covid-19 was too great, and every distortion of the truth was justified as necessary, not only to destroy HCQ, but Donald Trump’s mere well-intended recommendation of using HCQ for eligible patients.
This is how the anti-HCQ narrative was created. Everyone in charge seemed to mysteriously unify themselves in a coordinated “consensus” against HCQ.
In place of HCQ, new, minimally tested, expensive, extremely complex, rarely used gene therapy technology was proposed by Big Pharma, then unscientifically mandated by the Biden White House, and funded with obscene debt. The lies about HCQ and other repurposed treatments like ivermectin were promoted by the government and news organizations, making it seem like mRNA injections, unlimited boosters and novel, FDA-sanctioned therapies were only acceptable ways to prevent or treat Covid-19. The end product of the falsehoods and mRNA mandates adversely affected every single American citizen, with a very select few reaping astonishing profits, borne on the back of taxpayers.
Donald Trump and RFK’s recent collaboration and collaboration to Make America Healthy Again (MAHA) should eventually include a full investigation into blockaded HCQ as a repurposed treatment for Covid-19 in order to better understand its seemingly coordinated and wholly inappropriate condemnation.
It is no exaggeration to state that had Trump been allowed to proceed with his endeavor to distribute HCQ for Covid-19 in an appropriate patient population, we would be living in a much different United States of America. Today’s cumulative safety and efficacy findings on HCQ data are unequivocal proof detailing its benefits, particularly for the early treatment of Covid-19.
DISCLAIMER: Do NOT discontinue or initiate taking ANY drug without first discussing it with a pharmacist or physician you know and trust.
Dr. David Gortler is a pharmacologist and pharmacist. He is a former Yale University School of Medicine professor of pharmacology and biotechnology. While at Yale, he was recruited by the FDA to become a medical officer/senior medical analyst in the FDA’s Office of New Drugs. He was later appointed as senior advisor to the FDA commissioner on drug safety and FDA science policy. He is currently a senior fellow at the Heritage Foundation in Washington, DC, having previously served as a fellow at the Ethics and Public Policy Center.
Heritage is listed for identification purposes only. The views expressed in this article are the author’s own and do not reflect any institutional position for Heritage or its Board of Trustees.

No comments:
Post a Comment